Evolution of AAMI/ANSI Standards: Harmonizing with ISO

Good afternoon everyone. Almost six years ago, at three national dialysis conferences, I delivered a presentation with a topic title similar to this one.
If you attended one of those sessions, after we’re finished here, I think you’ll agree with the Greek philosopher that said… “The only constant in life is change.”
There is a lot of information to cover here, but I’ll try to move along quickly so that we can have a short question and answer period at the end of this session.
Copyright RPC - Vern Taaffe, 2018
If you attended one of those sessions, after we’re finished here, I think you’ll agree with the Greek philosopher that said… “The only constant in life is change.”
There is a lot of information to cover here, but I’ll try to move along quickly so that we can have a short question and answer period at the end of this session.
Copyright RPC - Vern Taaffe, 2018

Most of you are probably familiar with the acronyms AAMI, ANSI, and ISO.
For the sake of those not familiar, we’ll go through the next few slides quickly to explain how these organizations differ.
AAMI – is the acronym for “The Association for the Advancement of Medical Instrumentation”
It’s a nonprofit organization that was founded in 1967.
AAMI is a diverse community of nearly 7,000 healthcare technology professionals united by one important mission - supporting the healthcare community in the development, management, and use of safe and effective medical technology.
Copyright RPC - Vern Taaffe, 2018
For the sake of those not familiar, we’ll go through the next few slides quickly to explain how these organizations differ.
AAMI – is the acronym for “The Association for the Advancement of Medical Instrumentation”
It’s a nonprofit organization that was founded in 1967.
AAMI is a diverse community of nearly 7,000 healthcare technology professionals united by one important mission - supporting the healthcare community in the development, management, and use of safe and effective medical technology.
Copyright RPC - Vern Taaffe, 2018

AAMI is the primary resource in the United States for industry, the professions, and government… for national and international standards.
The AAMI standards program consists of over 100 technical committees and working groups. These committees and working groups produce Standards, Recommended Practices, and Technical Information Reports for medical devices, including dialysis devices and dialysis fluids.
The AAMI working group for dialysis is called the Renal Disease and Detoxification Committee or RDD Committee for short. The RDD committee meets in the spring and fall of each year to update existing standards, and to produce new standards, recommended practices, and Technical Information Reports.
Copyright RPC - Vern Taaffe, 2018

ANSI, is the acronym for the “American National Standards Institute”.
It is a federation that has served as coordinator and watchdog of the U.S. private sector voluntary standardization system for more than 90 years.
ANSI also accredits qualified organizations - whose standards development process meets all of ANSI’s requirements - to develop American National Standards. However, ANSI itself does not develop standards.
It’s important to note that, technically speaking, all AAMI/ANSI standards are voluntary guidelines.
Copyright RPC - Vern Taaffe, 2018
It is a federation that has served as coordinator and watchdog of the U.S. private sector voluntary standardization system for more than 90 years.
ANSI also accredits qualified organizations - whose standards development process meets all of ANSI’s requirements - to develop American National Standards. However, ANSI itself does not develop standards.
It’s important to note that, technically speaking, all AAMI/ANSI standards are voluntary guidelines.
Copyright RPC - Vern Taaffe, 2018

ISO, is the acronym for the “International Organization for Standardization”.
It is an independent, non-governmental international organization with a membership of 162 national standards bodies including the AAMI Renal Disease and Detoxification Committee representing the United States.
Through its members, it brings together experts to share knowledge and develop consensus-based, market relevant International Standards that support innovation and provide solutions to global challenges. ISO was founded in 1947 in Geneva, Switzerland.
Like the AAMI/ANSI Standards, ISO standards are also voluntary guidelines.
Copyright RPC - Vern Taaffe, 2018
It is an independent, non-governmental international organization with a membership of 162 national standards bodies including the AAMI Renal Disease and Detoxification Committee representing the United States.
Through its members, it brings together experts to share knowledge and develop consensus-based, market relevant International Standards that support innovation and provide solutions to global challenges. ISO was founded in 1947 in Geneva, Switzerland.
Like the AAMI/ANSI Standards, ISO standards are also voluntary guidelines.
Copyright RPC - Vern Taaffe, 2018

Originally, a Standard was intended to contain recommendations to the medical device manufacturer. Over time, the Standards have evolved to also be helpful to device purchasers or users to get an understanding of safe and effective device use and current technologies in a clinical environment.
A Recommended Practice is a set of guidelines mainly directed to the healthcare professional for the safe and effective use, care, and processing of a medical device.
Standards and recommended practices are subject to a formal process of committee approval, public review, and resolution of all comments. This process of consensus is supervised by the AAMI Standards Board and, in the case of American National Standards, by the American National Standards Institute.
A standard must be acted on—reaffirmed, revised, or withdrawn—and the action formally approved usually every five years, but at least every 10 years.
Copyright RPC - Vern Taaffe, 2018
A Recommended Practice is a set of guidelines mainly directed to the healthcare professional for the safe and effective use, care, and processing of a medical device.
Standards and recommended practices are subject to a formal process of committee approval, public review, and resolution of all comments. This process of consensus is supervised by the AAMI Standards Board and, in the case of American National Standards, by the American National Standards Institute.
A standard must be acted on—reaffirmed, revised, or withdrawn—and the action formally approved usually every five years, but at least every 10 years.
Copyright RPC - Vern Taaffe, 2018

An AAMI Recommended Practice that is accepted by ANSI becomes an ANSI/AAMI Standard.
All standards, recommended practices, technical information reports, and other types of technical documents developed by AAMI are voluntary, and their application is solely within the discretion and professional judgment of the user of the document.
Often, voluntary technical documents are adopted by government regulatory agencies or procurement authorities, in which case the adopting agency is responsible for enforcement of its rules and regulations.
Copyright RPC - Vern Taaffe, 2018
All standards, recommended practices, technical information reports, and other types of technical documents developed by AAMI are voluntary, and their application is solely within the discretion and professional judgment of the user of the document.
Often, voluntary technical documents are adopted by government regulatory agencies or procurement authorities, in which case the adopting agency is responsible for enforcement of its rules and regulations.
Copyright RPC - Vern Taaffe, 2018

A technical information report or TIR is an AAMI publication that addresses a particular aspect of medical technology. Dialysis related examples include a TIR on ultrapure dialysate released in 2011 and a TIR on water testing methodologies released in 2014.
Although the material presented in a TIR may need further evaluation by experts…releasing the information is valuable because the industry and the professions have an immediate need for it.
A TIR is not subject to the same formal approval process as a standard.
However, dialysis related TIRs are produced by and approved for distribution by the AAMI RDD Committee and the AAMI Standards Board.
For a dialysis related TIR, AAMI consults with its RDD Committee about five years after the publication date for guidance on whether the information in the document is still relevant or of historical value. If the information is not useful, the TIR is removed from circulation.
Unlike a standard, a TIR permits the inclusion of differing viewpoints on technical issues.
Copyright RPC - Vern Taaffe, 2018
Although the material presented in a TIR may need further evaluation by experts…releasing the information is valuable because the industry and the professions have an immediate need for it.
A TIR is not subject to the same formal approval process as a standard.
However, dialysis related TIRs are produced by and approved for distribution by the AAMI RDD Committee and the AAMI Standards Board.
For a dialysis related TIR, AAMI consults with its RDD Committee about five years after the publication date for guidance on whether the information in the document is still relevant or of historical value. If the information is not useful, the TIR is removed from circulation.
Unlike a standard, a TIR permits the inclusion of differing viewpoints on technical issues.
Copyright RPC - Vern Taaffe, 2018

Ok. Now that we know AAMI produces guidelines and standards, why is it that we should have standards for hemodialysis fluids?
We need the standards because patients are at risk of injury from contaminants in water and in the final dialysate. Some of these contaminants include aluminum, chloramines, fluoride, and microbial impurities.
In addition, contaminants are associated with morbidities. Some of these morbidities include anemia, bone disease, dialysis dementia, clinical complications such as pyrogenic reactions, septicemia, and inflammatory state associated with loss of kidney function
Copyright RPC - Vern Taaffe, 2018
We need the standards because patients are at risk of injury from contaminants in water and in the final dialysate. Some of these contaminants include aluminum, chloramines, fluoride, and microbial impurities.
In addition, contaminants are associated with morbidities. Some of these morbidities include anemia, bone disease, dialysis dementia, clinical complications such as pyrogenic reactions, septicemia, and inflammatory state associated with loss of kidney function
Copyright RPC - Vern Taaffe, 2018

Acknowledgement of these and other patient risks associated with dialysis fluids led to the development of the AAMI standards.
ANSI/AAMI RD5 for hemodialysis systems was the first dialysis standard. It was published in the United States in 1981 and was intended for dialysis equipment manufacturers. RD5 included dialysis machines, water treatment systems, concentrate and more.
Copyright RPC - Vern Taaffe, 2018
ANSI/AAMI RD5 for hemodialysis systems was the first dialysis standard. It was published in the United States in 1981 and was intended for dialysis equipment manufacturers. RD5 included dialysis machines, water treatment systems, concentrate and more.
Copyright RPC - Vern Taaffe, 2018
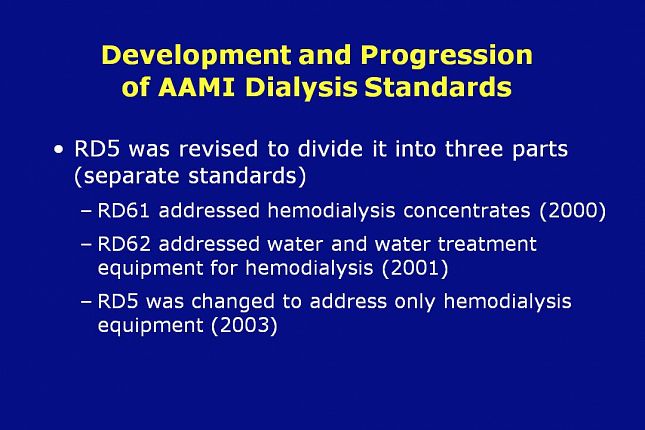
After multiple revisions for the purpose of updating RD5 over time, RD5 was ultimately revised to divide it into three separate standards.
In the year 2000, the hemodialysis concentrate part of RD5 was released as a separate standard identified as RD61. In 2001, the water and water treatment equipment part of RD5 was released as a separate standard identified as RD62…. and in 2003 RD5 was changed to address only hemodialysis equipment.
Copyright RPC - Vern Taaffe, 2018
In the year 2000, the hemodialysis concentrate part of RD5 was released as a separate standard identified as RD61. In 2001, the water and water treatment equipment part of RD5 was released as a separate standard identified as RD62…. and in 2003 RD5 was changed to address only hemodialysis equipment.
Copyright RPC - Vern Taaffe, 2018

This slide shows the first release of the RD62 water treatment document and the RD5 document after RD5 was divided into three separate standards.
Copyright RPC - Vern Taaffe, 2018
Copyright RPC - Vern Taaffe, 2018
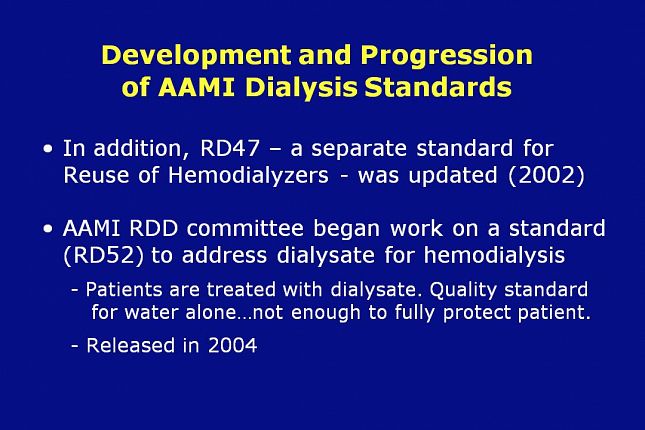
In addition to the separation of RD5 into three different standards, in 2002 – the RD47 standard for Reuse of Hemodialyzers was updated.
During the same time period, the AAMI RDD Committee began work on a standard to address dialysate for hemodialysis. Because the final fluid the patients are treated with is dialysate not water, the RDD committee recognized that the quality standard for water alone was not enough to fully protect the patient.
The dialysate standard was identified as RD52 and it was released in 2004.
Copyright RPC - Vern Taaffe, 2018
During the same time period, the AAMI RDD Committee began work on a standard to address dialysate for hemodialysis. Because the final fluid the patients are treated with is dialysate not water, the RDD committee recognized that the quality standard for water alone was not enough to fully protect the patient.
The dialysate standard was identified as RD52 and it was released in 2004.
Copyright RPC - Vern Taaffe, 2018

This slide shows the 2002 update of the RD47 Reuse of Hemodialyzers document and the 2004 first release of the RD52 Dialysate for Hemodialysis standard.
Copyright RPC - Vern Taaffe, 2018
Copyright RPC - Vern Taaffe, 2018

As stated earlier, AAMI standards are intended by AAMI to be voluntary.
The goal of the standards is to increase the safety and effectiveness of existing technologies and to support the development of new technologies.
The standards can provide device manufacturers with recommendations regarding basic safety and performance criteria and provide end users with guidelines for safe and effective device use.
Copyright RPC - Vern Taaffe, 2018
The goal of the standards is to increase the safety and effectiveness of existing technologies and to support the development of new technologies.
The standards can provide device manufacturers with recommendations regarding basic safety and performance criteria and provide end users with guidelines for safe and effective device use.
Copyright RPC - Vern Taaffe, 2018
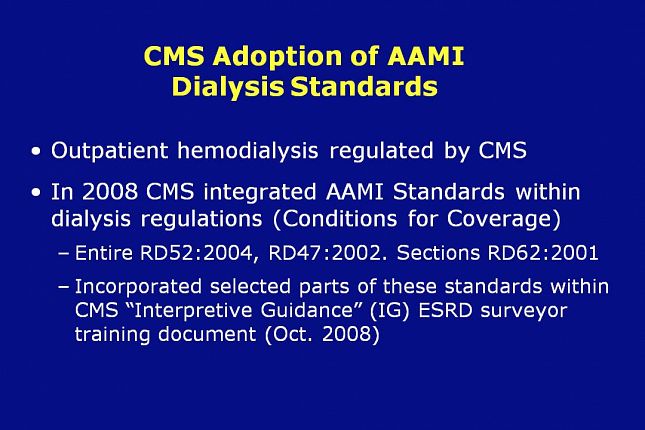
In the United States, outpatient hemodialysis is regulated by the Centers for Medicare and Medicaid Services.
In 2008, in their ESRD Conditions for Coverage, CMS adopted by reference the entire ANSI/AAMI RD52:2004 and RD47:2002 standards, and sections of RD62:2001.
As a result, compliance with all of the recommendations and guidelines in the respective AAMI standards is not voluntary; it’s mandatory.
For clarity, selected parts of these standards were incorporated, along with other CMS requirements, within the CMS “Interpretive Guidance” document used for ESRD surveyor training.
Copyright RPC - Vern Taaffe, 2018
In 2008, in their ESRD Conditions for Coverage, CMS adopted by reference the entire ANSI/AAMI RD52:2004 and RD47:2002 standards, and sections of RD62:2001.
As a result, compliance with all of the recommendations and guidelines in the respective AAMI standards is not voluntary; it’s mandatory.
For clarity, selected parts of these standards were incorporated, along with other CMS requirements, within the CMS “Interpretive Guidance” document used for ESRD surveyor training.
Copyright RPC - Vern Taaffe, 2018

This slide shows the CMS Interpretive Guidance document and the website address you can use to download it.
It’s likely that most of you already have this document in your clinics.
AAMI related sections that are included by CMS in the Interpretive Guidance cover water treatment equipment, water quality, dialysate quality, reuse practices, and monitoring practices for ensuring proper equipment operation and fluid quality.
Copyright RPC - Vern Taaffe, 2018
It’s likely that most of you already have this document in your clinics.
AAMI related sections that are included by CMS in the Interpretive Guidance cover water treatment equipment, water quality, dialysate quality, reuse practices, and monitoring practices for ensuring proper equipment operation and fluid quality.
Copyright RPC - Vern Taaffe, 2018

In 2009 and 2010, RD52, RD61, and RD62 - the AAMI standards that focus on hemodialysis fluids – and the dialysis fluid standards from ISO - all went through a major review. After these reviews and significant input from AAMI to ISO, AAMI decided to adopt a set of five dialysis fluid standards developed by the ISO.
The latest ISO standards now serve as replacements for the previous AAMI standards.
Copyright RPC - Vern Taaffe, 2018
The latest ISO standards now serve as replacements for the previous AAMI standards.
Copyright RPC - Vern Taaffe, 2018
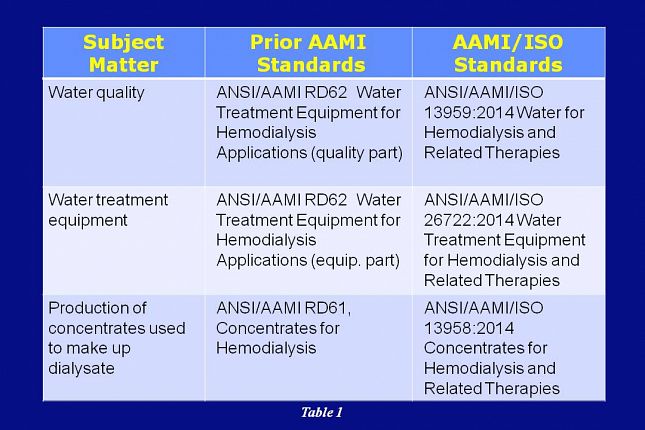
This table and the tables on the next slides provide a summary comparison of the AAMI adopted ISO standards and how they correlate with the previous AAMI standards.
Water quality was addressed in the prior AAMI RD62 standard and it is now being addressed in the AAMI/ISO 13959 standard.
Water treatment equipment was addressed in the prior AAMI RD62 standard and is now being addressed in the AAMI/ISO 26722 standard.
Production of concentrates was being addressed in the prior AAMI RD61 standard and it’s now being addressed in the AAMI/ISO 13958 standard.
Copyright RPC - Vern Taaffe, 2018
Water quality was addressed in the prior AAMI RD62 standard and it is now being addressed in the AAMI/ISO 13959 standard.
Water treatment equipment was addressed in the prior AAMI RD62 standard and is now being addressed in the AAMI/ISO 26722 standard.
Production of concentrates was being addressed in the prior AAMI RD61 standard and it’s now being addressed in the AAMI/ISO 13958 standard.
Copyright RPC - Vern Taaffe, 2018
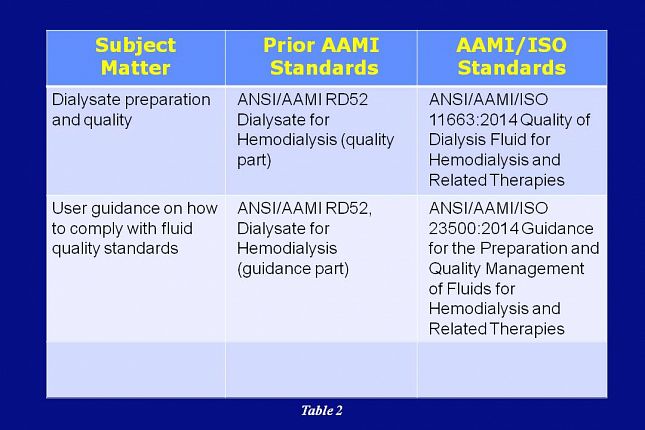
Dialysate preparation and quality was addressed in the prior AAMI RD52 standard and is now being addressed in the AAMI/ISO 11663 standard.
User guidance on compliance with fluid quality standards was also being addressed in the prior AAMI RD52 standard and it’s now being addressed in the AAMI/ISO 23500 standard.
The latest version release for each of these AAMI/ISO documents was in 2014.
Copyright RPC - Vern Taaffe, 2018
User guidance on compliance with fluid quality standards was also being addressed in the prior AAMI RD52 standard and it’s now being addressed in the AAMI/ISO 23500 standard.
The latest version release for each of these AAMI/ISO documents was in 2014.
Copyright RPC - Vern Taaffe, 2018
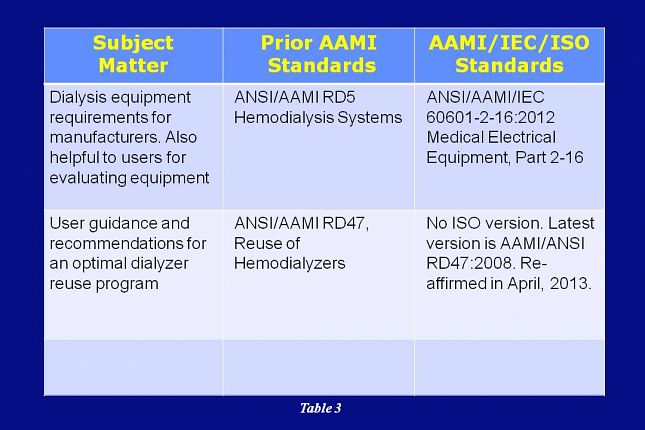
In addition to the five dialysis fluid standards just discussed, there are two other AAMI dialysis standards to consider. As mentioned earlier, RD5 evolved to cover only hemodialysis equipment.
Rather than an ISO standard, RD5 has been replaced by the 60601 standard developed by the International Electrotechnical Commission…or IEC for short.
AAMI RD47 covers user guidance and recommendations for an optimal dialyzer reuse program. The RD47 standard is not replaced by any other standard and the latest revision to it was in 2008.
In April of 2013, the AAMI RDD Committee evaluated RD47 and decided that it would be reaffirmed as is.
Copyright RPC - Vern Taaffe, 2018
Rather than an ISO standard, RD5 has been replaced by the 60601 standard developed by the International Electrotechnical Commission…or IEC for short.
AAMI RD47 covers user guidance and recommendations for an optimal dialyzer reuse program. The RD47 standard is not replaced by any other standard and the latest revision to it was in 2008.
In April of 2013, the AAMI RDD Committee evaluated RD47 and decided that it would be reaffirmed as is.
Copyright RPC - Vern Taaffe, 2018
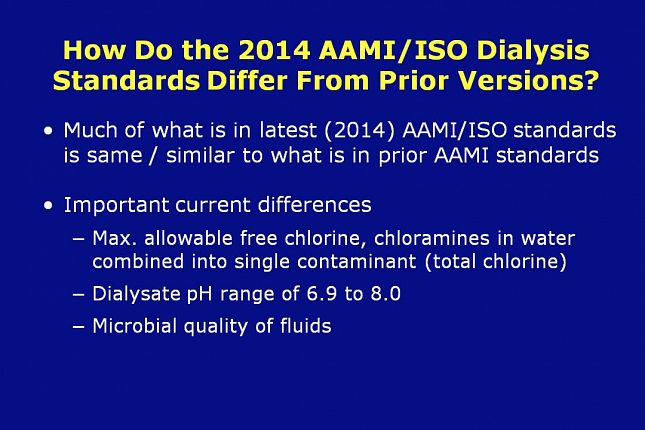
How do the latest AAMI/ISO standards differ from prior versions?
Much of the content in the AAMI adopted ISO standards should be familiar to anyone who has worked with the previous AAMI standards.
There are, however, some important differences. In the AAMI/ISO 13959 standard, the maximum allowable level of chemical contaminants in dialysis water is the same as in RD62:2006 except that free chlorine and chloramines have been combined into a single contaminant, total chlorine. The maximum allowable level of total chlorine is set at 0.1 ppm. This is the same value that RD62 listed for chloramines. In most dialysis facilities, total chlorine tests such as test strips are already being used to determine the level of chloramines in the pretreatment water. Therefore, this change in the new standard will have little or no impact.
The AAMI RD52 standard specifies a dialysate pH range of 6.9 to 7.6 while ISO specifies 6.9 to 8.0.
The more important differences are primarily in relation to the microbial quality of fluids. Let’s go to the next few slides to examine these differences.
Copyright RPC - Vern Taaffe, 2018
Much of the content in the AAMI adopted ISO standards should be familiar to anyone who has worked with the previous AAMI standards.
There are, however, some important differences. In the AAMI/ISO 13959 standard, the maximum allowable level of chemical contaminants in dialysis water is the same as in RD62:2006 except that free chlorine and chloramines have been combined into a single contaminant, total chlorine. The maximum allowable level of total chlorine is set at 0.1 ppm. This is the same value that RD62 listed for chloramines. In most dialysis facilities, total chlorine tests such as test strips are already being used to determine the level of chloramines in the pretreatment water. Therefore, this change in the new standard will have little or no impact.
The AAMI RD52 standard specifies a dialysate pH range of 6.9 to 7.6 while ISO specifies 6.9 to 8.0.
The more important differences are primarily in relation to the microbial quality of fluids. Let’s go to the next few slides to examine these differences.
Copyright RPC - Vern Taaffe, 2018
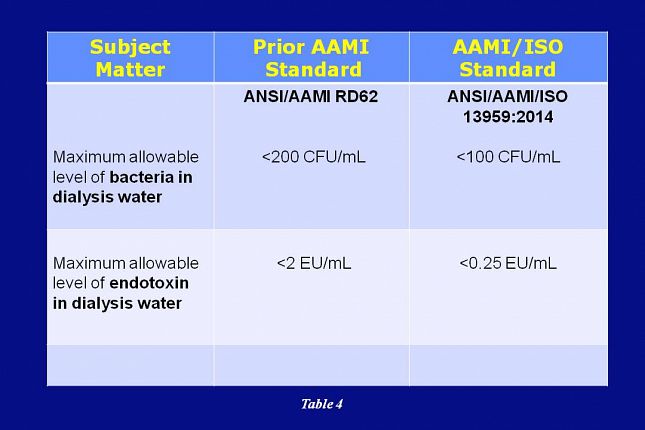
AAMI’s adoption of ISO 13959 reduces the allowable level of bacteria and endotoxin in dialysis water. The specification for bacteria is reduced from less than 200 CFU/mL to the new standard of less than 100 CFU/mL. Endotoxin is reduced from 2 EU/mL to the new standard of 0.25 EU/mL.
Copyright RPC - Vern Taaffe, 2018
Copyright RPC - Vern Taaffe, 2018
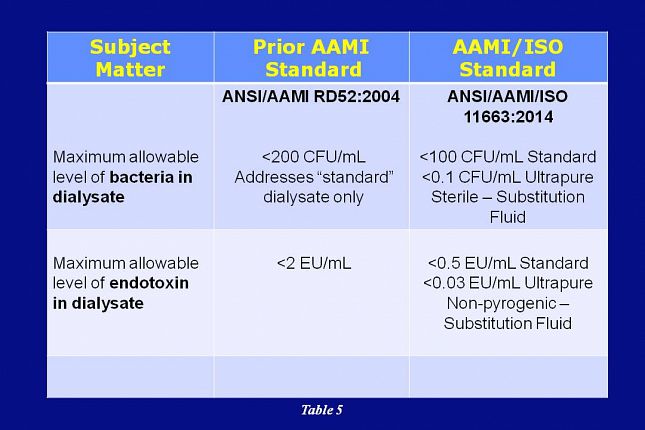
In addition, AAMI’s adoption of ISO 11663 acknowledges three levels of dialysate microbial quality: standard, ultrapure, and on-line prepared substitution fluid.
Standard quality dialysate is considered to be the minimum acceptable for clinical use.
To align the United States with other countries such as Europe and Japan, the ANSI/AAMI /ISO 23500 standard recommends the use of ultrapure dialysate for all treatments. Substitution fluid prepared on-line is intended for use in therapies such as hemofiltration and hemodiafiltration.
This slide shows that – in the ISO 11663 standard - the allowable level of bacteria in dialysate has been reduced to less than 100 CFU/mL for standard dialysate and less than 0.1 CFU/mL for ultrapure dialysate. Substitution fluid is required to be sterile and non-pyrogenic.
Also, the allowable level of endotoxin in dialysate has been reduced to less than 0.5 EU/mL for standard dialysate and less than 0.03 EU/mL for ultrapure dialysate.
Copyright RPC - Vern Taaffe, 2018
Standard quality dialysate is considered to be the minimum acceptable for clinical use.
To align the United States with other countries such as Europe and Japan, the ANSI/AAMI /ISO 23500 standard recommends the use of ultrapure dialysate for all treatments. Substitution fluid prepared on-line is intended for use in therapies such as hemofiltration and hemodiafiltration.
This slide shows that – in the ISO 11663 standard - the allowable level of bacteria in dialysate has been reduced to less than 100 CFU/mL for standard dialysate and less than 0.1 CFU/mL for ultrapure dialysate. Substitution fluid is required to be sterile and non-pyrogenic.
Also, the allowable level of endotoxin in dialysate has been reduced to less than 0.5 EU/mL for standard dialysate and less than 0.03 EU/mL for ultrapure dialysate.
Copyright RPC - Vern Taaffe, 2018
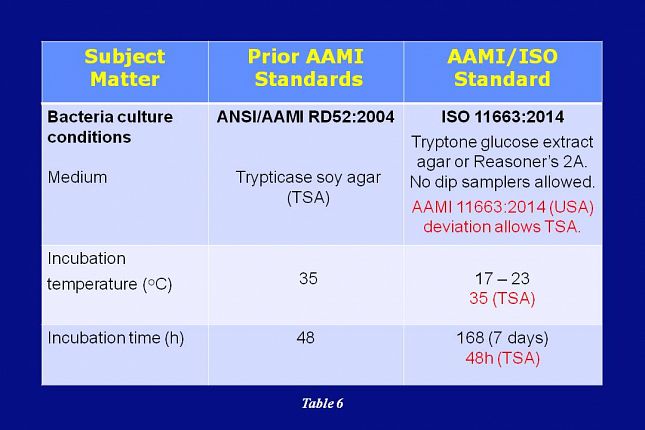
As shown on this slide, the ISO 11663 standard also specifies different media, incubation time, and incubation temperatures for bacterial cultures.
When compared with the RD52 TSA method, the new culturing conditions were thought to provide better recovery of bacteria from low nutrient fluids, such as dialysis water.
As a result, the AAMI RDD Committee initially accepted the new ISO culturing conditions.
However, after much discussion within the Committee, it was decided that for the United States, AAMI would deviate from ISO and allow the use of the TSA 35 degree C, 48 hour culture method. The RDD Committee reasoning for this deviation from ISO is explained on the next slide.
Copyright RPC - Vern Taaffe, 2018
When compared with the RD52 TSA method, the new culturing conditions were thought to provide better recovery of bacteria from low nutrient fluids, such as dialysis water.
As a result, the AAMI RDD Committee initially accepted the new ISO culturing conditions.
However, after much discussion within the Committee, it was decided that for the United States, AAMI would deviate from ISO and allow the use of the TSA 35 degree C, 48 hour culture method. The RDD Committee reasoning for this deviation from ISO is explained on the next slide.
Copyright RPC - Vern Taaffe, 2018

It’s important for patient safety to know sooner rather than later if the action level has been reached so that you can allow for earlier intervention
To that point, seven (7) days is a long time to wait for results
No culture method recovers all bacteria present
While higher recoveries may be ideal, shorter incubation methods allow for more rapid intervention
Many of the bacteria harmful to patients grow at higher temperatures, on higher nutrient medium and in a shorter time
How to establish equivalency to ISO 2014 recommended methods is not defined by ISO.
In addition, Drs Maltais and Meyer analyzed a large amount of lab data provided by USA dialysis providers and they determined that the TSA culture method is suitable for use in testing dialysis fluid samples.
To that point, seven (7) days is a long time to wait for results
No culture method recovers all bacteria present
While higher recoveries may be ideal, shorter incubation methods allow for more rapid intervention
Many of the bacteria harmful to patients grow at higher temperatures, on higher nutrient medium and in a shorter time
How to establish equivalency to ISO 2014 recommended methods is not defined by ISO.
In addition, Drs Maltais and Meyer analyzed a large amount of lab data provided by USA dialysis providers and they determined that the TSA culture method is suitable for use in testing dialysis fluid samples.

As a result of the RDD committee’s work, here are the changes AAMI proposed for the 2018 ISO Hemodialysis Standards.
- TSA incubated at 35-37° C, for 48h would be added as an acceptable culture method
Residual chlorine detection in rinse water following disinfection of a dialysis system with bleach per manufacturer’s instructions can be done by measuring for free chlorine at a limit of less than or equal to 0.5mg/L
The ISO documents should be consistent in the way the words “shall” and “should” are used.
- TSA incubated at 35-37° C, for 48h would be added as an acceptable culture method
Residual chlorine detection in rinse water following disinfection of a dialysis system with bleach per manufacturer’s instructions can be done by measuring for free chlorine at a limit of less than or equal to 0.5mg/L
The ISO documents should be consistent in the way the words “shall” and “should” are used.

Considering these AAMI change proposals, and input to ISO from it’s many other member countries , how will the updated 2018 ISO Standards differ from the 2014 versions?
Let’s take a look at what was decided by ISO.
The documents will be renumbered to form a dialysis specific group (#23500) with sub numbers (1-5) to define each document within the group.
The document group title is:
Guidance for the preparation and quality management of fluids for hemodialysis and related therapies
Copyright RPC - Vern Taaffe, 2018
Let’s take a look at what was decided by ISO.
The documents will be renumbered to form a dialysis specific group (#23500) with sub numbers (1-5) to define each document within the group.
The document group title is:
Guidance for the preparation and quality management of fluids for hemodialysis and related therapies
Copyright RPC - Vern Taaffe, 2018

This slide shows a cross reference for the existing ISO Standard document numbers and what each document’s new sub number will become within the 23500 document group.
Copyright RPC - Vern Taaffe, 2018
Copyright RPC - Vern Taaffe, 2018

The TSA 35° C, 48h culture method will be included - along with TGEA
and R2A 7 day methods - as acceptable culture methods.
The maximum allowable free chlorine rinse level will no longer be specified in the Standards.
- Manufacturers of dialysis machines or free chlorine tests are to call out the free
chlorine residual rinse level in their Instructions for Use.
- It’s highly likely that these manufacturers will continue with the free chlorine residual
rinse level unchanged at 0.5 mg/l (ppm)
-
Copyright RPC - Vern Taaffe, 2018
and R2A 7 day methods - as acceptable culture methods.
The maximum allowable free chlorine rinse level will no longer be specified in the Standards.
- Manufacturers of dialysis machines or free chlorine tests are to call out the free
chlorine residual rinse level in their Instructions for Use.
- It’s highly likely that these manufacturers will continue with the free chlorine residual
rinse level unchanged at 0.5 mg/l (ppm)
-
Copyright RPC - Vern Taaffe, 2018
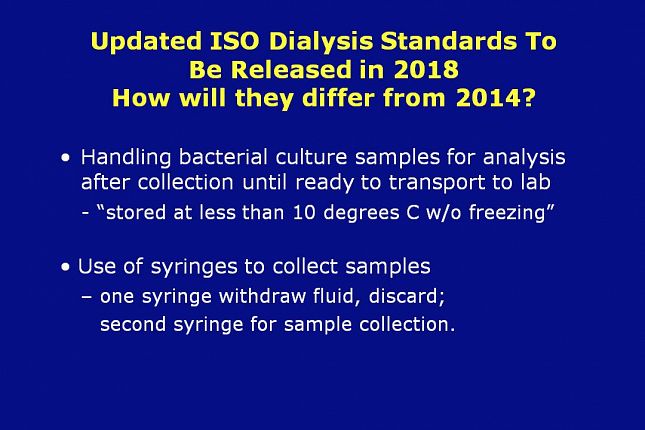
Other differences include:
Handling of bacterial culture samples for analysis from collection thru transport to lab
Microbial analysis of any fluid sample should be conducted as soon as possible after collection to avoid unpredictable changes in the microbial population. If samples cannot be analyzed within 4 h of collection, they should be stored at <10 °C without freezing until ready to transport to the laboratory for analysis. Sample storage for more than 24 h should be avoided, and sample shipping should be in accordance with the laboratory’s instructions.
Use of syringes to collect samples (depending on the type of sample port used)
– one syringe is used to withdraw fluid, discard; then a second syringe is used for sample collection.
Copyright RPC - Vern Taaffe, 2018
Handling of bacterial culture samples for analysis from collection thru transport to lab
Microbial analysis of any fluid sample should be conducted as soon as possible after collection to avoid unpredictable changes in the microbial population. If samples cannot be analyzed within 4 h of collection, they should be stored at <10 °C without freezing until ready to transport to the laboratory for analysis. Sample storage for more than 24 h should be avoided, and sample shipping should be in accordance with the laboratory’s instructions.
Use of syringes to collect samples (depending on the type of sample port used)
– one syringe is used to withdraw fluid, discard; then a second syringe is used for sample collection.
Copyright RPC - Vern Taaffe, 2018

So…does CMS require compliance with the latest AAMI/ISO dialysis standards?
In short, the answer is no, not at this time. CMS will continue to enforce the existing Conditions for Coverage for the foreseeable future. Therefore, until CMS updates its regulations, regulatory compliance will continue to be tied to the RD52:2004, RD62:2001, and RD47:2002 AAMI standards.
CMS is exploring adopting some elements of the ISO Standards; however, the time for this change process can be long.
This interim time period offers dialysis providers an opportunity to implement procedures that enable compliance with more stringent quality standards.
Copyright RPC - Vern Taaffe, 2018
In short, the answer is no, not at this time. CMS will continue to enforce the existing Conditions for Coverage for the foreseeable future. Therefore, until CMS updates its regulations, regulatory compliance will continue to be tied to the RD52:2004, RD62:2001, and RD47:2002 AAMI standards.
CMS is exploring adopting some elements of the ISO Standards; however, the time for this change process can be long.
This interim time period offers dialysis providers an opportunity to implement procedures that enable compliance with more stringent quality standards.
Copyright RPC - Vern Taaffe, 2018

Revision of existing CMS regulations requires a multiple step process, which explains the perceived lag between the AAMI RDD updates and possible changes to the Conditions for Coverage. The regulatory process includes information gathering from subject matter experts, in this case those from the dialysis technical field, as well as from the dialysis community regarding the impact such changes would have on the dialysis providers. If CMS determines that revisions to the Conditions for Coverage are indicated, a Notice of Proposed Rule would be published in the Federal Register, followed by a public comment period.
Although any such revision would not be expected to take as long, remember that the development process for the current regulations took more than 14 years.
It’s important to note that, in the interim, CMS does not discourage compliance with the more stringent new standards. Also, tag V181 in the Interpretive Guidance has the ultrapure dialysate specifications of RD52. While use of ultrapure dialysate is not currently a CMS requirement, if a facility states that they are delivering ultrapure dialysate, surveyors could hold that facility to those standards.
Copyright RPC - Vern Taaffe, 2018
Although any such revision would not be expected to take as long, remember that the development process for the current regulations took more than 14 years.
It’s important to note that, in the interim, CMS does not discourage compliance with the more stringent new standards. Also, tag V181 in the Interpretive Guidance has the ultrapure dialysate specifications of RD52. While use of ultrapure dialysate is not currently a CMS requirement, if a facility states that they are delivering ultrapure dialysate, surveyors could hold that facility to those standards.
Copyright RPC - Vern Taaffe, 2018

After many years of exceptional service, Judith Kari and Teri Spencer have retired from their work with CMS and the AAMI RDD Committee.
Contact information for the primary and alternate CMS representatives – that will replace Judith and Teri on the AAMI Committee - is shown on this slide.
Copyright RPC - Vern Taaffe, 2018
Contact information for the primary and alternate CMS representatives – that will replace Judith and Teri on the AAMI Committee - is shown on this slide.
Copyright RPC - Vern Taaffe, 2018

The information on this slide was provided by Jennifer Milby of CMS to the AAMI RDD Committee in November of 2017. CMS’ current actions in regard to dialysis include:
Finalizing the Dialysis in Nursing Home survey process and guidance with an anticipated release within the next couple of months
Working on revising the State Operations Manual-Appendix H to update the content and survey process to be consistent with the core survey process and the 2008 Conditions for Coverage.
Updating the guidance for recent Survey and Certification memos , including
Filling saline syringes at the patient station (S&C 17-31) and
Cleaning the patient station (S&C 17-32) and
Revising the frequently asked questions document (FAQ’s)
- This FAQ document has been removed from the CMS website until the update is complete
Copyright RPC - Vern Taaffe, 2018
Finalizing the Dialysis in Nursing Home survey process and guidance with an anticipated release within the next couple of months
Working on revising the State Operations Manual-Appendix H to update the content and survey process to be consistent with the core survey process and the 2008 Conditions for Coverage.
Updating the guidance for recent Survey and Certification memos , including
Filling saline syringes at the patient station (S&C 17-31) and
Cleaning the patient station (S&C 17-32) and
Revising the frequently asked questions document (FAQ’s)
- This FAQ document has been removed from the CMS website until the update is complete
Copyright RPC - Vern Taaffe, 2018
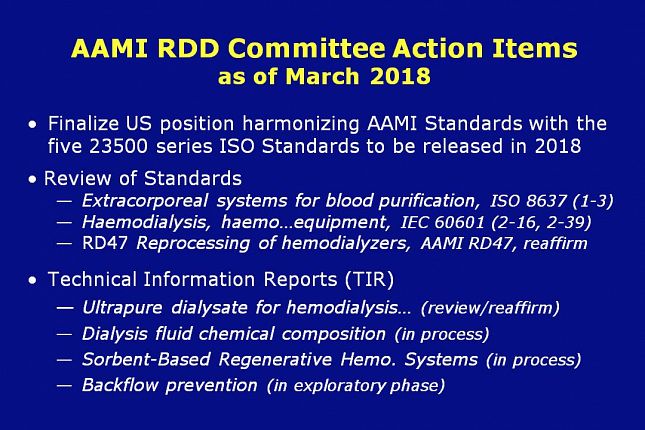
This slide shows the AAMI RDD committee action items that are in process as of today.
The RDD committee is finalizing the US position harmonizing AAMI Standards with the five 23500 series ISO Standards that will be released this year.
We are reviewing the ISO Standard on Extracorporeal systems for blood purification, the IEC Standard on Hemo. Equipment, and the AAMI Standard on Reprocessing of hemodialyzers. The RD47 Reprocessing Standard of 2008 was reaffirmed “as is” in 2014. It’s likely that will happen again in 2018.
In addition, several Technical Information Reports are under review or development.
The TIR reports in process include a review of the 2011 document on Ultrapure dialysate, and new TIRs that are shown here. The TIRs on dialysis fluid composition and on Sorb systems have both been submitted to the AAMI Standards Board for approval (a formality).
Copyright RPC - Vern Taaffe, 2018
The RDD committee is finalizing the US position harmonizing AAMI Standards with the five 23500 series ISO Standards that will be released this year.
We are reviewing the ISO Standard on Extracorporeal systems for blood purification, the IEC Standard on Hemo. Equipment, and the AAMI Standard on Reprocessing of hemodialyzers. The RD47 Reprocessing Standard of 2008 was reaffirmed “as is” in 2014. It’s likely that will happen again in 2018.
In addition, several Technical Information Reports are under review or development.
The TIR reports in process include a review of the 2011 document on Ultrapure dialysate, and new TIRs that are shown here. The TIRs on dialysis fluid composition and on Sorb systems have both been submitted to the AAMI Standards Board for approval (a formality).
Copyright RPC - Vern Taaffe, 2018

Let’s conclude and summarize this presentation:
AAMI produces voluntary Standards, Recommended Practices, and TIRs for medical devices
Prior versions of AAMI standards for dialysis fluids have been adopted by CMS as regulatory requirements
Current AAMI standards for dialysis fluids will be harmonized with ISO standards in 2018
Compliance with the latest AAMI/ISO standards is not yet required by CMS, and
AAMI continues its unending work of updating existing standards, while developing new standards & TIRs
Copyright RPC - Vern Taaffe, 2018
AAMI produces voluntary Standards, Recommended Practices, and TIRs for medical devices
Prior versions of AAMI standards for dialysis fluids have been adopted by CMS as regulatory requirements
Current AAMI standards for dialysis fluids will be harmonized with ISO standards in 2018
Compliance with the latest AAMI/ISO standards is not yet required by CMS, and
AAMI continues its unending work of updating existing standards, while developing new standards & TIRs
Copyright RPC - Vern Taaffe, 2018

Thank you very much for attending this session.
At this time, please feel free to ask any questions that you may have.
Copyright RPC - Vern Taaffe, 2018
At this time, please feel free to ask any questions that you may have.
Copyright RPC - Vern Taaffe, 2018
