Dialyzer Reuse: Exploding the Myths
Download (.zip)
Presented by Vern Taaffe at the 2006 IDS National Technical Meeting in Long Beach, California.
Good morning and thank you for attending this session.
Objectives for the session are:
1) To identify misconceptions associated with dialyzer reuse, that have emerged over time
2) To dispel these misconceptions through an examination of the facts, and
3) To review critical dialyzer reuse quality control steps
Please write down any questions you may have during the session.
There will be some time to answer questions at the end of the session.
To stay on time, we may go through some of the slides quickly.
Copies of the slides can be found in your speaker handouts.
Good morning and thank you for attending this session.
Objectives for the session are:
1) To identify misconceptions associated with dialyzer reuse, that have emerged over time
2) To dispel these misconceptions through an examination of the facts, and
3) To review critical dialyzer reuse quality control steps
Please write down any questions you may have during the session.
There will be some time to answer questions at the end of the session.
To stay on time, we may go through some of the slides quickly.
Copies of the slides can be found in your speaker handouts.

What is a “Myth”?
Merriam-Webster dictionary defines a “myth” as:
• a popular belief or tradition that has grown up around something or someone
• a person or thing having only an imaginary or unverifiable existence
There are certain popular beliefs — that have grown up around dialyzer reuse — beliefs that have only an imaginary or unverifiable existence.
Merriam-Webster dictionary defines a “myth” as:
• a popular belief or tradition that has grown up around something or someone
• a person or thing having only an imaginary or unverifiable existence
There are certain popular beliefs — that have grown up around dialyzer reuse — beliefs that have only an imaginary or unverifiable existence.

In the last few years, the following misconceptions have grown up around dialyzer reuse:
• patient safety is compromised
• validated dialyzer maximum use limit is not necessary
• bacteria cannot grow in refrigerated dialyzers
• no medical benefit for the patient, and there is
• no economic gain for the dialysis provider
Starting with patient safety, let’s examine each of these misconceptions.
• patient safety is compromised
• validated dialyzer maximum use limit is not necessary
• bacteria cannot grow in refrigerated dialyzers
• no medical benefit for the patient, and there is
• no economic gain for the dialysis provider
Starting with patient safety, let’s examine each of these misconceptions.

Here are the facts associated with patient safety when measured by Mortality Risk – Reuse vs. No-Reuse:
According to the latest studies by Port, Ebben, and an NKF task force, mortality was not greater in reuse units when compared with non-reuse units.
More factors were considered in these more recent studies (compared with earlier studies).
The factors include comorbidity, disease severity, hematocrit, etc.
In addition, the Centers for Medicare & Medicaid Services Dialysis Center Compare Report for the year 2000 shows that 10 of the top 12 dialysis units reused dialyzers.
It is also clear from these and and other studies- that you can expect an increase in mortality risk if you have an ineffective QA program.
According to the latest studies by Port, Ebben, and an NKF task force, mortality was not greater in reuse units when compared with non-reuse units.
More factors were considered in these more recent studies (compared with earlier studies).
The factors include comorbidity, disease severity, hematocrit, etc.
In addition, the Centers for Medicare & Medicaid Services Dialysis Center Compare Report for the year 2000 shows that 10 of the top 12 dialysis units reused dialyzers.
It is also clear from these and and other studies- that you can expect an increase in mortality risk if you have an ineffective QA program.

Over the past 30 years, many researchers have studied various aspects of performance and quality control for reused hemodialyzers. Most of the researchers have shown that dialyzer reuse can be a safe and effective practice when appropriate quality control measures are in place…. However, dialyzer reuse will not be safe — just as a dialysis treatment will not be safe — if performed improperly, or if there is a lack of appropriate quality control.
QC measures are not likely to be as effective if implemented without consideration for high risk problems.
High risk problems are defined as problems that may result in patient injury or death.
Specific high risk problems have re-occurred periodically in different dialysis centers throughout the U.S.
At times some of these problems have been incorrectly associated with dialyzer reprocessing.
An example of an effective six step QA program is outlined on the next several slides
QC measures are not likely to be as effective if implemented without consideration for high risk problems.
High risk problems are defined as problems that may result in patient injury or death.
Specific high risk problems have re-occurred periodically in different dialysis centers throughout the U.S.
At times some of these problems have been incorrectly associated with dialyzer reprocessing.
An example of an effective six step QA program is outlined on the next several slides

Here are the six primary steps:
Understand and comply with the Association for Advancement of Medical Instrumentation and Centers for Medicare & Medicaid Services requirements.
Develop a written reuse “method-specific” policy and procedures.
Identify “high risk” patient problems for prevention.
Set a validated maximum use limit for dialyzers.
Qualify and select an appropriate test lab for dialysis samples.
Report death, serious injury or illness, attributable to a medical device to FDA and/or the manufacturer of the device.
Let’s take a look at each of these steps:
Understand and comply with the Association for Advancement of Medical Instrumentation and Centers for Medicare & Medicaid Services requirements.
Develop a written reuse “method-specific” policy and procedures.
Identify “high risk” patient problems for prevention.
Set a validated maximum use limit for dialyzers.
Qualify and select an appropriate test lab for dialysis samples.
Report death, serious injury or illness, attributable to a medical device to FDA and/or the manufacturer of the device.
Let’s take a look at each of these steps:

No doubt most of you are familiar with these two documents or at least the document on the left which is the RD47 AAMI/ANSI Standard for Reuse of Hemodialyzers, originally released in May of 1993, with a revised version released in November of 2002. It will be up for review in 2007.
The document on the right is the Centers for Medicare & Medicaid Services (CMS) comprehensive form used by ESRD surveyors when evaluating a dialysis center’s operations, including the reuse program.
The CMS document incorporates the AAMI reuse standard, and a few additional requirements, in a checklist form, as conditions for compliance.
The document on the right is the Centers for Medicare & Medicaid Services (CMS) comprehensive form used by ESRD surveyors when evaluating a dialysis center’s operations, including the reuse program.
The CMS document incorporates the AAMI reuse standard, and a few additional requirements, in a checklist form, as conditions for compliance.
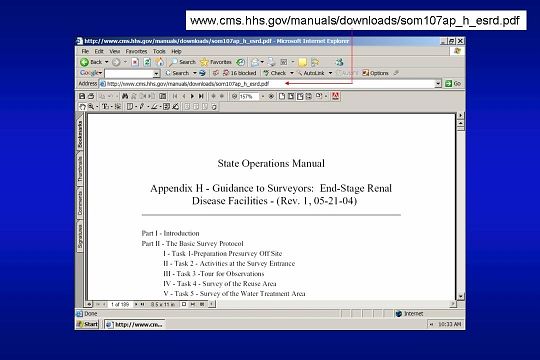
The CMS document is entitled: GUIDANCE TO SURVEYORS: END-STAGE RENAL DISEASE FACILITIES. It is Appendix H of the State Operations Manual. The most recent version can be downloaded from the CMS Web site as shown here.
The first step in implementing or maintaining an effective QA program is to read, understand and comply with the requirements listed in the AAMI and CMS documents.
The first step in implementing or maintaining an effective QA program is to read, understand and comply with the requirements listed in the AAMI and CMS documents.

Compliance with AAMI and CMS can be simplified by summarizing and listing the key requirements of the documents:
Develop a Dialyzer Reprocessing Manual (this was originally referred to as the Master Record).
Compile a history record that includes label information from each reprocessing.
Create written procedures for documenting control of safety and performance.
Make a form & file for recording complaints or incidents and corrective action taken
Develop periodic audit procedures and a schedule for conducting audits.
Identify and establish written procedures for compliance with the Occupational Safety and Health Administration and any additional state requirements that may be applicable.
The first five items can be traced directly to AAMI’s use of the Food & Drug Administration’s Good Manufacturing Practices — now known as the Quality System Regulations or QSRs.
Medical device manufacturers are required to comply with the FDA QSRs.
Develop a Dialyzer Reprocessing Manual (this was originally referred to as the Master Record).
Compile a history record that includes label information from each reprocessing.
Create written procedures for documenting control of safety and performance.
Make a form & file for recording complaints or incidents and corrective action taken
Develop periodic audit procedures and a schedule for conducting audits.
Identify and establish written procedures for compliance with the Occupational Safety and Health Administration and any additional state requirements that may be applicable.
The first five items can be traced directly to AAMI’s use of the Food & Drug Administration’s Good Manufacturing Practices — now known as the Quality System Regulations or QSRs.
Medical device manufacturers are required to comply with the FDA QSRs.

Now let’s review the development of a reuse “method-specific” policy. A written dialyzer reprocessing or reuse policy should include but is not limited to:
A reference to the AAMI/CMS documents as guidelines for the reprocessing program.
A policy on informed consent.
A policy on dialyzers from positive patients.
The title of the person or persons in charge of the reprocessing program and training.
General procedures specific to the reprocessing method in use.
The location of all detailed procedures, records, forms and documents.
The Medical Director’s signature of approval on the policy.
A procedure for reporting death, injury or serious illness as required by the SMDA - 1990.
A reference to the AAMI/CMS documents as guidelines for the reprocessing program.
A policy on informed consent.
A policy on dialyzers from positive patients.
The title of the person or persons in charge of the reprocessing program and training.
General procedures specific to the reprocessing method in use.
The location of all detailed procedures, records, forms and documents.
The Medical Director’s signature of approval on the policy.
A procedure for reporting death, injury or serious illness as required by the SMDA - 1990.

The words “method-specific”, used in reference to the reuse policy, mean the type of system and germicide you are using to reprocess dialyzers. Most of the systems in use are shown on this slide and include:
• Renal Systems’ Renatron and Renatron II.
• the Fresenius DRS-4.
• Mesa Medicals’ Echo.
• Two newer machines: HDC Medical’s Maky and the Alcavis ARM machine.
• Manual systems.
• Heat disinfection with citric acid.
Typical germicides in use include formaldehyde, gluteraldehyde, and peracetic acid-based germicides such as Micro-X®, Renalin, and Peracidin.
• Renal Systems’ Renatron and Renatron II.
• the Fresenius DRS-4.
• Mesa Medicals’ Echo.
• Two newer machines: HDC Medical’s Maky and the Alcavis ARM machine.
• Manual systems.
• Heat disinfection with citric acid.
Typical germicides in use include formaldehyde, gluteraldehyde, and peracetic acid-based germicides such as Micro-X®, Renalin, and Peracidin.

The next step in the QA process is to identify high risk patient problems for prevention.
In general these problems — that can be associated with dialyzer reprocessing — are:
• Pyrogenic Reactions & Bacteremia.
• Allergic Reactions.
• Blood Leaks.
• Particulate in Dialyzers.
• Inadequate Dialysis.
An in-depth examination of each problem and its solution requires more time than we have available today. However, in 1992, I wrote a comprehensive guideline article entitled “Preventing Specific High Risk Problems Typically Associated with Dialyzer Reprocessing”. I’ve updated it four times since 1992 with the most recent update in December of 2002. The guideline format is to list each problem, and its solution or solutions, along with the rationale. Edition 2 of the Amgen Dialysis Technician Core Curriculum references this guideline.
It can be downloaded, free-of-charge, from the Technical Support Information section of the RPC Web site. The Web site address will be displayed on the last slide of this presentation.
In general these problems — that can be associated with dialyzer reprocessing — are:
• Pyrogenic Reactions & Bacteremia.
• Allergic Reactions.
• Blood Leaks.
• Particulate in Dialyzers.
• Inadequate Dialysis.
An in-depth examination of each problem and its solution requires more time than we have available today. However, in 1992, I wrote a comprehensive guideline article entitled “Preventing Specific High Risk Problems Typically Associated with Dialyzer Reprocessing”. I’ve updated it four times since 1992 with the most recent update in December of 2002. The guideline format is to list each problem, and its solution or solutions, along with the rationale. Edition 2 of the Amgen Dialysis Technician Core Curriculum references this guideline.
It can be downloaded, free-of-charge, from the Technical Support Information section of the RPC Web site. The Web site address will be displayed on the last slide of this presentation.

Confirmed sources of these problems are shown on this slide. Endotoxins and bacteria in the water treatment system continue to be at the top of the list for causing patient problems. Therefore, proper maintenance and disinfection of the water system is essential.
Cleaning agent or germicide mixing errors have also been the source of pyrogenic reactions, bacteremia, and allergic reactions.
Forces which physically stress the dialyzer are present during the reprocessing procedure, during the pre-dialysis rinse procedure, and during the treatment. Water quality, pressure, flow rate, temperature, and PH change — along with cleaner and germicide exposure — can contribute to dialyzer material degradation over time.
In addition, clotting of dialyzer fibers during the treatment can result in a cumulative decrease to the dialysis dose and inadequate dialysis.
Cleaning agent or germicide mixing errors have also been the source of pyrogenic reactions, bacteremia, and allergic reactions.
Forces which physically stress the dialyzer are present during the reprocessing procedure, during the pre-dialysis rinse procedure, and during the treatment. Water quality, pressure, flow rate, temperature, and PH change — along with cleaner and germicide exposure — can contribute to dialyzer material degradation over time.
In addition, clotting of dialyzer fibers during the treatment can result in a cumulative decrease to the dialysis dose and inadequate dialysis.

Additional considerations for minimizing patient risk are shown on this slide and the next slide.
Psychrophilic bacteria can grow in refrigerated dialyzers.
Psychrophilic bacteria is an organism which grows optimally at or below 15°C. It has an upper limit for growth at approximately 20°C, and has a lower limit of growth of 0°C or lower.
Examples are gram negative Yersinia enterocolitica, and gram positive Listeria monocytogenes.
In a morbidity and mortality report dated June 20,1997, the Centers for Disease Control reported on 10 cases of Yersinia enterocolitica sepsis, associated with transfusions with contaminated blood, from refrigerated blood bags … five of the ten patients died.
Reuse germicides would be expected to kill this bacteria; however, endotoxin problems may result.
Also, refrigeration may reduce the number of times a dialyzer can be reused as blood solidifies in the dialyzer.
Consider the alternative: pre-cleaning with germicide dwell; it flushes out blood and the germicide prevents growth.
To date, I’m not aware of any reports of patient problems that have been directly associated with dialyzer refrigeration. However, it may be difficult to identify or link bacteria or endotoxin problems with refrigeration.
It would make sense to adjust your procedures to minimize the length of time a dialyzer must be refrigerated.
Psychrophilic bacteria can grow in refrigerated dialyzers.
Psychrophilic bacteria is an organism which grows optimally at or below 15°C. It has an upper limit for growth at approximately 20°C, and has a lower limit of growth of 0°C or lower.
Examples are gram negative Yersinia enterocolitica, and gram positive Listeria monocytogenes.
In a morbidity and mortality report dated June 20,1997, the Centers for Disease Control reported on 10 cases of Yersinia enterocolitica sepsis, associated with transfusions with contaminated blood, from refrigerated blood bags … five of the ten patients died.
Reuse germicides would be expected to kill this bacteria; however, endotoxin problems may result.
Also, refrigeration may reduce the number of times a dialyzer can be reused as blood solidifies in the dialyzer.
Consider the alternative: pre-cleaning with germicide dwell; it flushes out blood and the germicide prevents growth.
To date, I’m not aware of any reports of patient problems that have been directly associated with dialyzer refrigeration. However, it may be difficult to identify or link bacteria or endotoxin problems with refrigeration.
It would make sense to adjust your procedures to minimize the length of time a dialyzer must be refrigerated.

Another factor to be aware of is that:
Direct use of full-strength germicide, in reuse machines, may differ from use of externally mixed germicides.
With regard to the final germicide delivered to the dialyzer: Results from an RPC/independent lab study showed increased germicide variability at reuse machine low end water pressure — and a higher average concentration of the germicide — with direct use of full-strength germicide in a popular reuse machine. The results are in comparison with the same germicide formulation — when externally mixed — for use with the same type of reuse machine. An application note summary of the study can be downloaded from the RPC Web site.
Why is this important?
It’s important because inadequate germicide mixing and/or higher germicide concentrations may increase rinse time, increase the risk for allergic reactions, and lead to other problems.
To date, I am not aware of any reports of patient problems directly associated with reuse machines using full-strength germicide. However, if your reuse machine mixing relies mainly on water pressure then it would seem prudent to use optimum water pressure and carefully monitor dialyzer rinse results.
Direct use of full-strength germicide, in reuse machines, may differ from use of externally mixed germicides.
With regard to the final germicide delivered to the dialyzer: Results from an RPC/independent lab study showed increased germicide variability at reuse machine low end water pressure — and a higher average concentration of the germicide — with direct use of full-strength germicide in a popular reuse machine. The results are in comparison with the same germicide formulation — when externally mixed — for use with the same type of reuse machine. An application note summary of the study can be downloaded from the RPC Web site.
Why is this important?
It’s important because inadequate germicide mixing and/or higher germicide concentrations may increase rinse time, increase the risk for allergic reactions, and lead to other problems.
To date, I am not aware of any reports of patient problems directly associated with reuse machines using full-strength germicide. However, if your reuse machine mixing relies mainly on water pressure then it would seem prudent to use optimum water pressure and carefully monitor dialyzer rinse results.
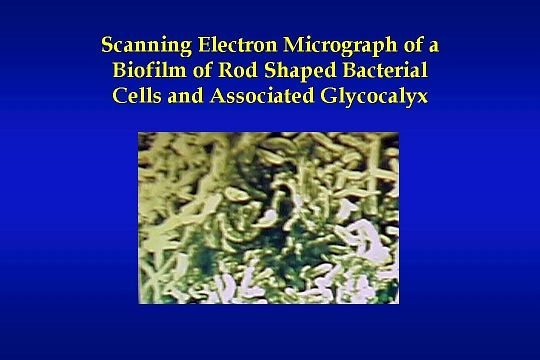
This slide shows a graphic example of one cause for the pyrogenic reactions and bacteremic episodes identified earlier. It is a scanning electron micrograph of a biofilm of rod-shaped bacterial cells and their associated glycocalyx. Dissection of a piece of water system piping and an electronically enlarged photograph of the pipe allows us to see an actual bacterial biofilm that has formed on the inner surface of the piping with time.
This is the type of problem that can develop in a water treatment system without proper design, maintenance, and routine disinfection. Without routine disinfection — which is defined as once a month minimum — a dialysis unit may experience normal water system cultures for a long period of time. However, the biofilm accumulates until part of it eventually breaks free into the central water stream causing a catastrophic increase in bacteria delivered to the dialysis machines and reprocessing equipment. This problem and other “high risk” problems listed earlier can be avoided through an understanding of the source of the problems and implementation of proven quality control measures, to prevent the problems.
This is the type of problem that can develop in a water treatment system without proper design, maintenance, and routine disinfection. Without routine disinfection — which is defined as once a month minimum — a dialysis unit may experience normal water system cultures for a long period of time. However, the biofilm accumulates until part of it eventually breaks free into the central water stream causing a catastrophic increase in bacteria delivered to the dialysis machines and reprocessing equipment. This problem and other “high risk” problems listed earlier can be avoided through an understanding of the source of the problems and implementation of proven quality control measures, to prevent the problems.

The next step in the QA process is to set a maximum use limit for dialyzers. The number selected for each model dialyzer should be based on a documented analysis of proper dialyzer performance for each successive use. This is part of the requirement for documented process control. In addition to the total cell volume and pressure integrity performance test, that are common in reprocessing equipment, the analysis should include verification that the maximum use limit selected is not set so high that it contributes to deterioration of the dialyzer or inadequate dialysis.
If you are interested in additional details, or an in-depth examination, on the need to set and validate a maximum use limit for dialyzers, please refer to an article I wrote entitled “Quality Assurance for Hemodialyzer Reprocessing: Minimizing Risks”. You can find it in the June 2001 issue of Nephrology News & Issues or download it from the RPC Web site.
If you are interested in additional details, or an in-depth examination, on the need to set and validate a maximum use limit for dialyzers, please refer to an article I wrote entitled “Quality Assurance for Hemodialyzer Reprocessing: Minimizing Risks”. You can find it in the June 2001 issue of Nephrology News & Issues or download it from the RPC Web site.

In the December 1997 issue of the American Journal of Kidney Diseases, an NKF task force released a comprehensive report on dialyzer reuse. One element of this still valid report was to point out the performance changes that can occur in different dialyzer types relative to the type of cleaner or germicide used and other multiple use factors.
This slide shows the various component parts of a dialyzer that can be altered by the reuse process causing the changes in performance cited by the NKF task force.
• The potting compound can deteriorate and develop holes, distortion, and particulate.
• Case cracks may result in particulate, leaks, or appearance problems.
• The fiber bundle may change in symmetry and increase in pore size … changing the KUF.
• Header O-rings and caps can trap bacteria or cause blood leaks.
The FDA requires manufacturers to label their dialyzers for reuse if they intend to sell them to centers that will reuse them. To receive approval, the manufacturer must provide test information which supports their reuse labeling. Therefore, if you are using dialyzers labeled for reuse, the manufacturer should be able to provide you with a suggested maximum use number that will minimize problems due to dialyzer material degradation.
This slide shows the various component parts of a dialyzer that can be altered by the reuse process causing the changes in performance cited by the NKF task force.
• The potting compound can deteriorate and develop holes, distortion, and particulate.
• Case cracks may result in particulate, leaks, or appearance problems.
• The fiber bundle may change in symmetry and increase in pore size … changing the KUF.
• Header O-rings and caps can trap bacteria or cause blood leaks.
The FDA requires manufacturers to label their dialyzers for reuse if they intend to sell them to centers that will reuse them. To receive approval, the manufacturer must provide test information which supports their reuse labeling. Therefore, if you are using dialyzers labeled for reuse, the manufacturer should be able to provide you with a suggested maximum use number that will minimize problems due to dialyzer material degradation.

While I’ve listed qualification and selection of an appropriate test lab as number 5 of the 6 steps, it certainly is higher than number 5 in terms of importance. Many of the “high risk” problems I’ve helped dialysis centers solve over the past 30 years could either have been prevented or more easily identified had the labs in use provided accurate bacteria reports.
The AAMI recommendations are shown on this slide.
Failure to follow these procedures may, and probably will, result in inadequate or inaccurate bacterial test information for making clinical QA decisions.
The AAMI recommendations are shown on this slide.
Failure to follow these procedures may, and probably will, result in inadequate or inaccurate bacterial test information for making clinical QA decisions.

This slide shows the sixth step listed in the QA process.
Make sure a written procedure exists for reporting incidents of death, serious injury, or illness that can be attributed to a medical device. This action is in accordance with the FDA’s Medical Device User Reporting procedures.
FDA’s definition of a medical device includes, but is not limited to, dialysis water systems, dialysis machines, reprocessing equipment & cleaners, and germicides used in the reprocessing procedure.
Make sure a written procedure exists for reporting incidents of death, serious injury, or illness that can be attributed to a medical device. This action is in accordance with the FDA’s Medical Device User Reporting procedures.
FDA’s definition of a medical device includes, but is not limited to, dialysis water systems, dialysis machines, reprocessing equipment & cleaners, and germicides used in the reprocessing procedure.

Questions about mandatory reporting can be answered by the FDA Division of Surveillance Systems, Reporting Systems Branch.
There is no need to write down the information as this slide is in your handouts.
FDA, CDRH, MDR User Reporting P.O. Box 3002 Rockville, MD 20847-3002
301-594-2735 301-827-0038 (fax)
There is no need to write down the information as this slide is in your handouts.
FDA, CDRH, MDR User Reporting P.O. Box 3002 Rockville, MD 20847-3002
301-594-2735 301-827-0038 (fax)
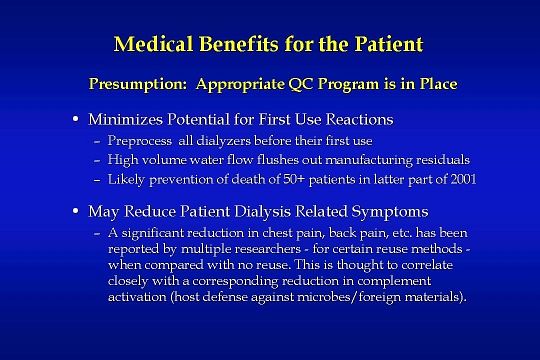
Dialyzer reuse certainly does provide medical benefits for the patient:
Preprocessing of all dialyzers before their first use minimizes the potential for a patient to have an allergic first-use reaction. High-volume water flow from the reuse equipment flushes out manufacturing residuals. In the latter part of 2001, more than 50 patients died as a result of a manufacturing agent present in Baxter dialyzers. The residual agent was PF-5070, a 3M brand fluid used to check for leaks in dialyzers. An examination of the MSDS sheet for this fluid makes it clear that the PF-5070 residuals could have been rinsed from the dialyzers had the dialyzers been preprocessed as part of a dialyzer reuse program.
In addition, dialyzer reuse has been shown to reduce patient dialysis related symptoms
A significant reduction in chest pain, back pain, etc. has been reported by multiple researchers — for certain reuse methods — when compared with no reuse. This is thought to correlate closely with a corresponding reduction in complement activation which is a host defense against microbes and foreign materials.
Preprocessing of all dialyzers before their first use minimizes the potential for a patient to have an allergic first-use reaction. High-volume water flow from the reuse equipment flushes out manufacturing residuals. In the latter part of 2001, more than 50 patients died as a result of a manufacturing agent present in Baxter dialyzers. The residual agent was PF-5070, a 3M brand fluid used to check for leaks in dialyzers. An examination of the MSDS sheet for this fluid makes it clear that the PF-5070 residuals could have been rinsed from the dialyzers had the dialyzers been preprocessed as part of a dialyzer reuse program.
In addition, dialyzer reuse has been shown to reduce patient dialysis related symptoms
A significant reduction in chest pain, back pain, etc. has been reported by multiple researchers — for certain reuse methods — when compared with no reuse. This is thought to correlate closely with a corresponding reduction in complement activation which is a host defense against microbes and foreign materials.

Significant economic gain — independent of new dialyzer cost —- is a definite benefit of dialyzer reuse.
The “Reused Dialyzer Cost Formula” shown on this slide can be used to determined the amount of money saved by reusing dialyzers.
Each variable in the formula is defined here and applied in the examples on some of the following slides.
The “Reused Dialyzer Cost Formula” shown on this slide can be used to determined the amount of money saved by reusing dialyzers.
Each variable in the formula is defined here and applied in the examples on some of the following slides.
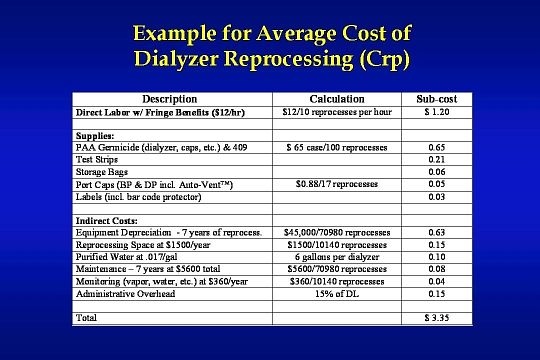
Here is an example for an average cost of dialyzer reprocessing totaling $ 3.35.
Competition among vendors has driven down the cost of dialyzer reprocessing supplies over the last several years. Supply costs shown in this example may actually be lower today.
Competition among vendors has driven down the cost of dialyzer reprocessing supplies over the last several years. Supply costs shown in this example may actually be lower today.

An example of the annual savings from reusing high cost dialyzers is shown here.
A new dialyzer price of $29, an average number of uses at 17, a $3.35 cost of reprocessing from the prior slide, and an average clinic size of 65 patients, were used to complete the calculations in the “Reused Dialyzer Cost Formula”.
An annual savings of $240,825 certainly qualifies as a significant economic benefit for the dialysis provider. In addition, a corresponding reduction in waste is an environmental gain that will benefit everyone.
A new dialyzer price of $29, an average number of uses at 17, a $3.35 cost of reprocessing from the prior slide, and an average clinic size of 65 patients, were used to complete the calculations in the “Reused Dialyzer Cost Formula”.
An annual savings of $240,825 certainly qualifies as a significant economic benefit for the dialysis provider. In addition, a corresponding reduction in waste is an environmental gain that will benefit everyone.

When the new dialyzer price is lowered to $9.50, the annual savings are still significant at approximately $55,000. In addition, the reduction of waste is an important environmental benefit that should not be overlooked.
So why is it that the largest clinical provider of dialysis services has converted many of their dialysis centers to non-reuse? Perhaps the answer lies in the fact that the company is also a dialyzer manufacturer. In the November 2002 issue of Nephrology News & Issues, Dr. J. Michael Lazarus, Senior Vice President and Medical Director of FMCNA, made the following statement: “I don’t think there’s anything wrong with reuse. I’ve supported it and defended it for 25 years … the major issue was that the cost of reuse was just about as much as the cost of the dialyzer. But this was somewhat of a gamble for us.” It is important to note that the Fresenius cost for the dialyzer is considerably less than your cost for the same dialyzer.
In a June 2001 presentation at NephroAsia, Dr. Lazarus also made the following statement: “Reuse of dialyzers is an option to be considered when resources for providing dialysis therapy are limited…. If reuse makes the difference in using a large surface area high-flux biocompatible dialyzer, then it is the opinion of this presenter that reuse should be employed”.
So why is it that the largest clinical provider of dialysis services has converted many of their dialysis centers to non-reuse? Perhaps the answer lies in the fact that the company is also a dialyzer manufacturer. In the November 2002 issue of Nephrology News & Issues, Dr. J. Michael Lazarus, Senior Vice President and Medical Director of FMCNA, made the following statement: “I don’t think there’s anything wrong with reuse. I’ve supported it and defended it for 25 years … the major issue was that the cost of reuse was just about as much as the cost of the dialyzer. But this was somewhat of a gamble for us.” It is important to note that the Fresenius cost for the dialyzer is considerably less than your cost for the same dialyzer.
In a June 2001 presentation at NephroAsia, Dr. Lazarus also made the following statement: “Reuse of dialyzers is an option to be considered when resources for providing dialysis therapy are limited…. If reuse makes the difference in using a large surface area high-flux biocompatible dialyzer, then it is the opinion of this presenter that reuse should be employed”.

To wrap up this session, the following question must be asked:
Do you still give consideration to one or more of these dialyzer reuse myths?
If you do, then please visit the technical support section of the RPC Web site for additional educational information you can use to dispel misconceptions associated with dialyzer reuse.
Thank you very much for your attention and interest in this session.
At this point, there is time to take any questions you may have.
Do you still give consideration to one or more of these dialyzer reuse myths?
If you do, then please visit the technical support section of the RPC Web site for additional educational information you can use to dispel misconceptions associated with dialyzer reuse.
Thank you very much for your attention and interest in this session.
At this point, there is time to take any questions you may have.
